Q.
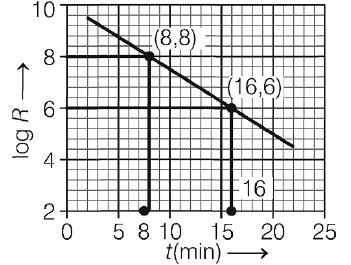
The graph shows the log of activity $\log \,R$ of a radioactive material as a function of time $t$ in minutes

The half-life (in minute) for the decay is closest to
KVPYKVPY 2018Nuclei
Solution: