Q.
The figure shows two paths that may be taken by a gas from an initial point $i$ to final point $f$. Path $1$ consists of an isothermal expansion (work is $50 \, J$ in magnitude), an isothermal compression (work is $30 \, J$ in magnitude), an adiabatic expansion (work is $40\, J$ in magnitude) and then an adiabatic compression (work is $25\, J$ in magnitude). Find the magnitude of change in internal energy (in $ J$) in path $2$, i.e., in path $AFGE$.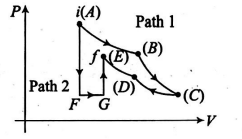
Thermodynamics
Solution: