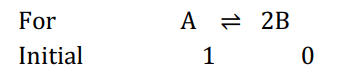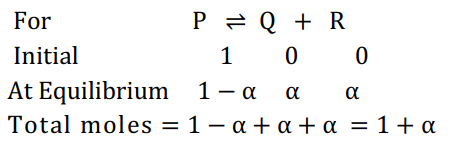Q. The equilibrium constants $K_{p_1}$ and $K_{p_2}$ for the reactions $A \rightleftharpoons 2 B$ and $P \rightleftharpoons Q + R$, respectively, are in the ratio of $2: 3$. If the degree of dissociation of $A$ and $P$ are equal, the ratio of the total pressure at equilibrium is,
Equilibrium
Solution: