Q.
The equilibrium constant for a reversible chemical reaction varies with $T$ as:
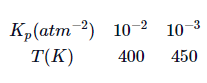
From this, it may be deduced that:
J & K CETJ & K CET 2001
Solution: