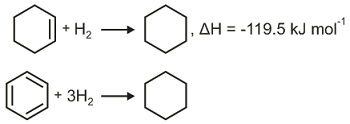
Q. The enthalpy of hydrogenation of cyclohexene is $-119.5\, kJ\, mol ^{-1}$. If resonance energy of benzene is $-150.4\, kJ\, mol ^{-1}$, its enthalpy of hydrogenation would be
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: