Q.
The diagram shows the energy levels for an electron in a certain atom. Which transition
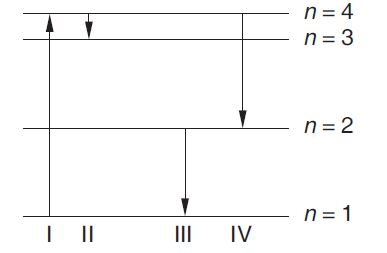
shown represents the emission of a photon with the most energy ?
UPSEEUPSEE 2007
Solution: