Q.
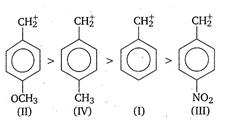
The descending order of stability, of the carbonium ions
$ C _{6} H _{5} \overset{+}{C} H _{2}( I ), p\left( CH _{3} O \right) C _{6} H _{4} \overset{+}{C} H _{2}( II ), p\left( NO _{2}\right) C _{6} H _{4} \overset{+}{C} H _{2}( III )$ and $p\left( CH _{3}\right) C _{6} H _{4} \overset{+}{ C } H _{2}( IV )$
J & K CETJ & K CET 2009Organic Chemistry – Some Basic Principles and Techniques
Solution:
In the presence of electron releasing group, (like $ CH_{3},CH_{3}O $ ), the positive charge is dispersed more. This dispersal of positive charge stabilises the carbocation (carbonium ion). While electron withdrawing substituent (like $NO_2$ ) destabilises the carbonium ion by intensifying the positive charge. Thus, the order of stability of given benzyl carbonium ions is as