Q.
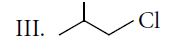
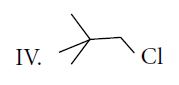
The decreasing order of SN2 reaction for the given compounds is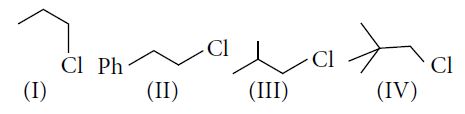
Solution:
Rate of SN2 reaction $\propto$ -I-effect, - M-effect
$\quad\quad\quad\quad\quad\quad\quad\propto\frac{1}{steric \,crowding}$
$I.\quad CH_{3} - CH_{2} - CH_{2} - Cl$
$II.\quad Ph - CH_{2} - CH_{2} - Cl$
Thus, rate of SN2 reaction is II > I > III > IV.