Q.
The correct statements among (a) to (b) are:
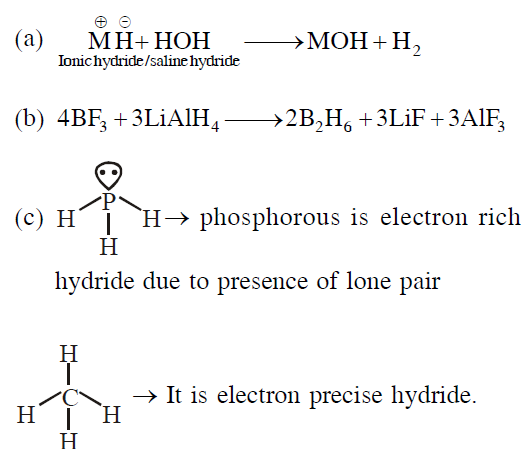
(a) saline hydrides produce $H_2$ gas when reacted with $H_2O$.
(b) reaction of $LiAH_4$ with $BF_3$ leads to $B_2H_6$.
(c) $PH_3$ and $CH_4$ are electron - rich and electronprecise hydrides, respectively.
(d) $HF$ and $CH_4$ are called as molecular hydrides.
Solution:
(d) $HF \& CH_4$ are molecular hydride due to they are covalent molecules.