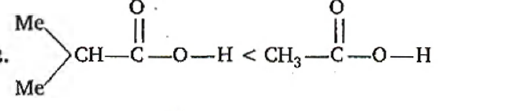Q.
The correct order of increasing acid strength of the compounds :
(A)$CH_{2} CO_{2} H$
(B) $MeOCH_{2}CO_{2}H$
(C) $CF_{3} CO_{2}H$
(D)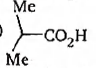
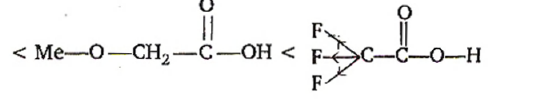
AIEEEAIEEE 2008
Solution:
-I effect increases acidity. +I effect decreases acidity. -$CF_{3}$ exerting more -I effect than MeO - $Me_{2}$CH- exerting more +I effect than —$CH_{3}$