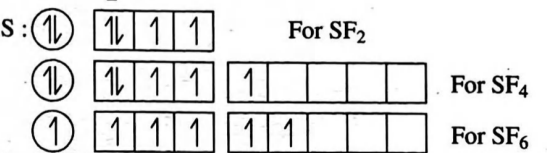
Q. The correct hybridization state of sulphur atom in $SF _{2}$, $SF _{4},$ and $SF _{6}$ molecules is respectively:
Chemical Bonding and Molecular Structure
Solution:
Correct answer is (b) $s p^{3}, s p^{3} d, s p^{3} d^{2}$