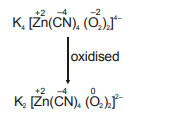Q. The complex $K _{4}\left[ Zn ( CN )_{4}\left( O _{2}\right)_{2}\right]$ is oxidised into $K _{2}\left[ Zn ( CN )_{4}\left( O _{2}\right)_{2}\right]$, then which of the following is/are correct -
Coordination Compounds
Solution: