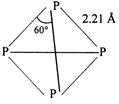
Q. The atomicity of phosphorus island the PPP bond angle in the molecule is Y. What are X and Y
EAMCETEAMCET 2003
Solution:
White phosphorus has the molecular formula $ {{\text{P}}_{4}} $ both in solid and vapour state at moderate temperature. The four atoms present in the molecule are arranged at the comers of a tetrahedro so, the PPP bond angle is $ \text{6}{{\text{0}}^{\text{o}}}. $ At higher temperature (above $ \text{700}{{\,}^{\text{o}}}\text{C} $ ), it dissociates to give diatomic molecules as: $ {{P}_{4}}\rightleftharpoons 2{{P}_{2}} $ Structure of $ {{\text{P}}_{\text{4}}} $