Q.
The arrangement of $ X^- $ ions around $ A^+ $ ion in
solid $AX$ is given in the figure (not drawn to scale). If the
radius of $ X^- $ is $250\, pm$, the radius of $ A^+ $ is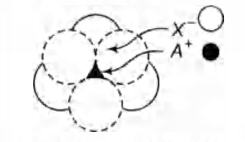
Solution: