Q.
Study the following graphs and choose the correct option
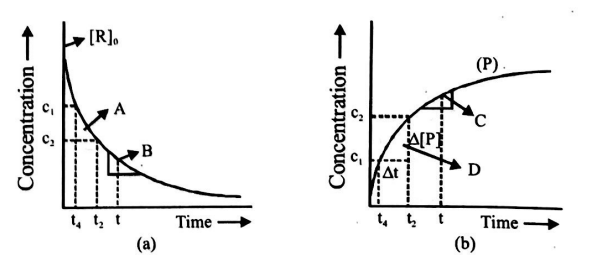
(i) in fig.$ a, A$ represents average rate and $B$ represents instantaneous rate
(ii) in fig. $b, D$ represents average rate and $C$ represents instantaneous rate
(iii) fig. $a, A$ represents instantaneous rate and $B$ represents average rate
(iv) fig. $b, C$ represents average rate and $D$ represents instantaneous rate
Chemical Kinetics
Solution: