Q.
Study the figure given below and mark the correct expression.
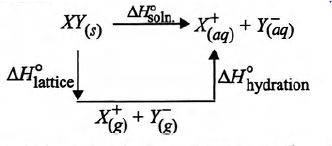
The enthalpy of solution of $XY_{(s)}$, $\Delta H^°_{soln.}$ in water can be determined by
Thermodynamics
Solution: