Q.
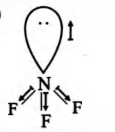
Statement 1 : $NF_3$ has little tendency to act as a donor
molecule.
Statement 2 : The highly electronegative $F$ atoms attract electrons and these moments partly cancel the moment from the lone pair.
Chemical Bonding and Molecular Structure
Solution: