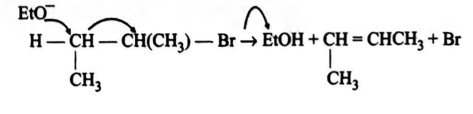Q.
Statement 1: $2$-Bromobutane on reaction with sodium
ethoxide in ethanol gives $2$-butene as a major product.
Statement 2: $2$-Butene is more stable than $1$-butene.
Hydrocarbons
Solution: