Q. Show the coordination number of the metal ion, its oxidation number, the number of electrons ind-orbitals and the number of unpaired electrons in d-orbitals respectively in complex $[Co(H_2O)_4SO_3 ]Cl.$
Gujarat CETGujarat CET 2010Coordination Compounds
Solution:
Coordination number is the number of monodentate ligands in the coordination sphere. Hence, the coordination number of cobait ion in $[Co(H_2O)_4 SO_3 ]Cl is 6.$
Let the oxidation number of Co is x.
x + 4(0) + (-2) + (-1) = 0
x + 0 - 2 - 1 = 0
x=3
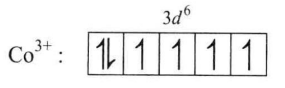
Number of eiectrons in d-orbital are six
Number of unpaired eiectrons in d-orbitaI are 4 because $H_2O$ is a weak ligand and therefore, pairing of d-electrons in d-orbital is not possibie.