Q.
Paragraph 4
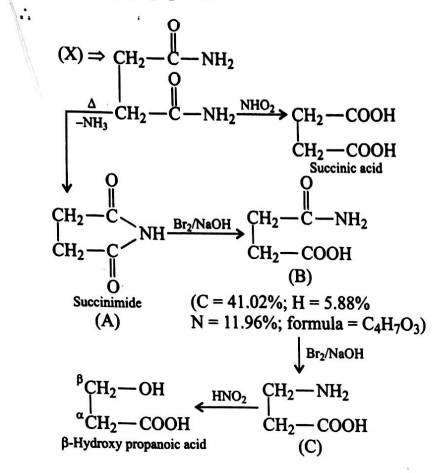
A substance (X) contains $41.37 \%$ C, $6.89 \%$ H. $0.116\, gm$ of $(X)$ gave $NH _{3}$, which was absorbed in $50\, ml$ of $N / 10 H _{2} SO _{4}$. The excess of acid required $30 ml$ of $N / 10\, NaOH$ for neutralisation. (X) on treatment with $HNO _{2}$ gave succinic acid. (X) on heating lost $NH _{3}$ to give (A). (A) reacts with $Br _{2}$ and $NaOH$ to give (B) containing $41.02 \% C , 5.88 \% H$, and $11.96 \% N$. (B) on further treatment with $Br _{2}$ and $NaOH$ gives (C) (3-amino propanoic acid). (C) reacts with $HNO _{2}$ to give $\beta$-hydroxy-propanoic acid.
Compound (C) is:
Amines
Solution: