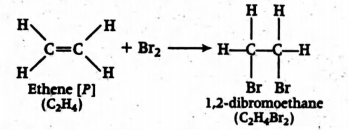Q.
$[P]\xrightarrow{Br_2} C_2H_4Br_2 \xrightarrow[NH_3] {NaNH_2}[Q]$
$ [Q]\xrightarrow[Hg^{2+} , \Delta ]{20 \% H_2 SO_4} [R] \xrightarrow{Zn-Hg/HCl} [S] $
The species P, Q, R and S respectively are
Solution: