Q.
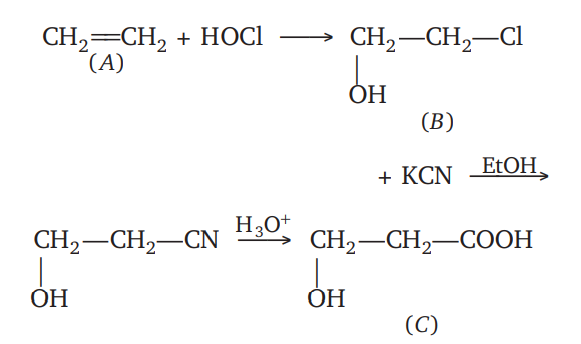
One per cent composition of an organic compound $A$ is, carbon : $85.71 \%$ and hydrogen $14.29 \%$. Its vapour density is $14$ . Consider the following reaction sequence

VITEEEVITEEE 2009
Solution: