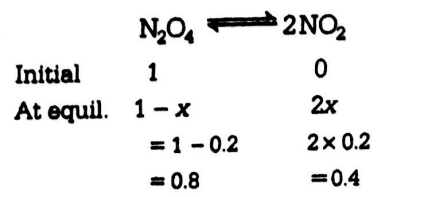Q. One mole of $ N_2O_{4} (g) $ at $ 300 K $ is kept in a closed vessel at $ 1 \, atm $ pressure. It is heated to $ 600 K $ when $ 20\% $ by mass of $ N_2O_4(g) $ decomposes to $ NO_2(g) $ . The resultant pressure is
AMUAMU 2015Equilibrium
Solution: