Q.
One mole of monatomic ideal gas undergoes the thermodynamic process $a \rightarrow b$ as shown in the $P-V$ diagram. During this process, the maximum temperature will occur when gas volume is $\frac{m}{n} V_{0} .$ Find $(m+n)$.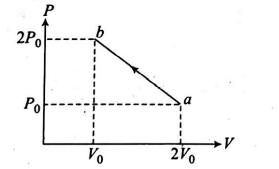
Thermodynamics
Solution: