Q.
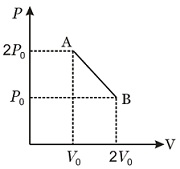
$n$ moles of an ideal gas undergoes a process $A \rightarrow B$ as shown in the diagram. The maximum temperature of the gas during the process is
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: