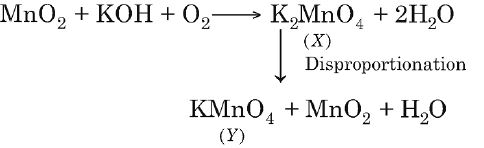Q. $MnO_{2}$ when fused with $KOH$ and oxidised in air gives a dark green compound $X$. In acidic solution, $X$ undergoes disproportionation to give an intense purple compound $Y$ and $MnO_{2}$. The compounds $X$ and $Y,$ respectively, are
KVPYKVPY 2015Redox Reactions
Solution: