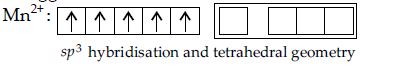
Q. $Mn^{2+}$ forms a complex with $Br^-$ ion. The magnetic moment of the complex is 5.92 BM. The probable formula and geometry of the complex, is
Coordination Compounds
Solution:
Magnetic moment $= 5.92\, BM =\sqrt{n\left(n+2\right)}$
$n = 5 =$ no. of unpaired electrons.
It suggests