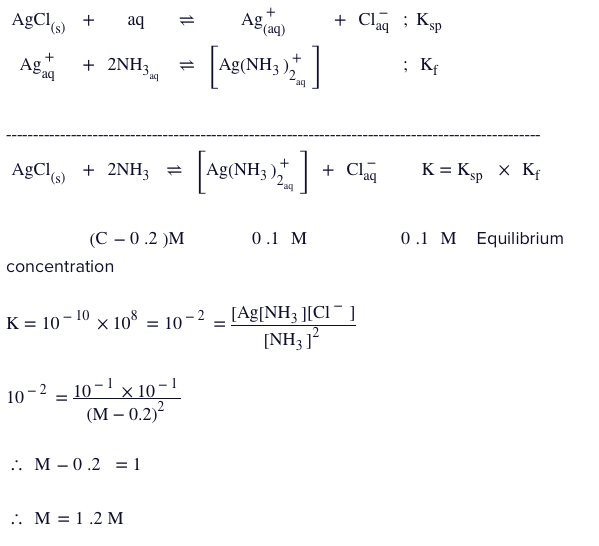
Q.
Minimum moles of $NH _{3}$ required to be added to $1 L$ solution so as to dissolve $0.1 mol$ of $AgCl \left( K _{ sp }=1.0 \times 10^{-10}\right)$ by the reaction is:
$
AgCl ( s )+2 NH _{3} \rightleftharpoons\left[ Ag \left( NH _{3}\right)_{2}\right]^{+}+ Cl ^{-}
$
Given $K _{ f }$ of $\left[ Ag \left( NH _{3}\right)_{2}\right]^{+}=10^{8}$
NTA AbhyasNTA Abhyas 2022
Solution: