Q.
Match the column.
Column I
Column II
1
$SF_2$
p
$sp^3$ and bent
2
$XeF_4$
q
Two lone pairs on central metal atom
3
$NOCl$
r
Bond angle $< 109.5^{\circ}$
4
$NF_3$
s
$sp^2$ and bent
t
$sp^3 d^2$ and square planar
| Column I | Column II | ||
|---|---|---|---|
| 1 | $SF_2$ | p | $sp^3$ and bent |
| 2 | $XeF_4$ | q | Two lone pairs on central metal atom |
| 3 | $NOCl$ | r | Bond angle $< 109.5^{\circ}$ |
| 4 | $NF_3$ | s | $sp^2$ and bent |
| t | $sp^3 d^2$ and square planar | ||
NTA AbhyasNTA Abhyas 2022
Solution:
 $\theta < 109.208$ (bent)
$\theta < 109.208$ (bent)  (Square planar)
(Square planar)  (bent)
(bent) 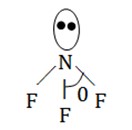 (Pyramidal)
(Pyramidal)