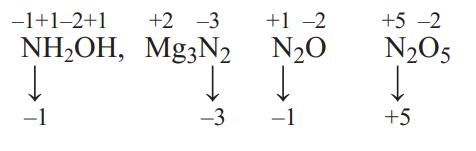Q.
Match the Column I with Column II and select the correct option for oxidation number of $N$-atom from the codes given below.
Column I(Compounds)
Column II(Oxidation number)
A
$NH_{2} OH$
1
$-1$
B
$Mg_3N_{2}$
2
$-1$
C
$N_2O$
3
$+5$
D
$N_2O _{5}$
4
$-3$
| Column I(Compounds) | Column II(Oxidation number) | ||
|---|---|---|---|
| A | $NH_{2} OH$ | 1 | $-1$ |
| B | $Mg_3N_{2}$ | 2 | $-1$ |
| C | $N_2O$ | 3 | $+5$ |
| D | $N_2O _{5}$ | 4 | $-3$ |
Redox Reactions
Solution:
The correct match is $A \rightarrow 2, B \rightarrow 4, C \rightarrow 1, D \rightarrow 3$ The oxidation number of $N$-atom in given compounds are shown below: