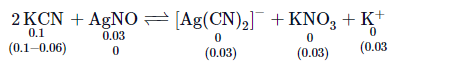Q. $K_{c}$ for the the reaction, $\left[ Ag ( CN )_{2}\right]^{-} \rightleftharpoons Ag ^{+}+2 CN ^{-}$, the equillibrium constant at $25^{\circ} C$ is $4.0 \times 10^{-19}$, then the silver ion concentration in a solution which was originally $0.1$ molar in $KCN$ and $0.03$ molar in $AgNO _{3}$ is :
BITSATBITSAT 2014
Solution: