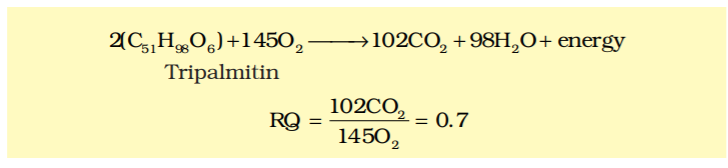Q.
In the given equation below, identify the compound which is being oxidized and calculate the R.Q. of the same.
$2\left(C_{51} H_{98} O_{6}\right)+145O_{2} \rightarrow 102CO_{2}+98H_{2}O+energy$
NTA AbhyasNTA Abhyas 2020Respiration in Plants
Solution: