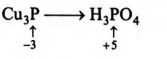Q.
In the following unbalanced redox reaction,
$Cu_{3}P+Cr_{2}O^{2-}_{7} \to Cu^{2+}+H_{3}PO_{4}+Cr^{3+}$
Equivalent weight of $H_{3}PO_{4}$ is
Redox Reactions
Solution: