Q.
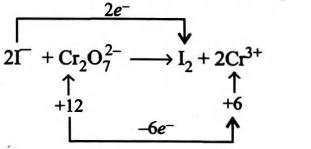
In the following reaction,
$2I - + Cr_{2}O^{2-}_{7} + 14H^{+} \to I_{2} + 2CI_{3+} + 7H_{2}0$
Unbalanced parts are
Redox Reactions
Solution: