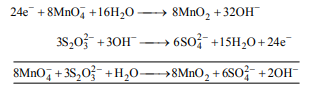
Q. In neutral or faintly alkaline solution, $8$ moles of permanganate anion quantitatively oxidize thiosulphate anions to produce $X$ moles of a sulphur containing product. The magnitude of $X$ is
JEE AdvancedJEE Advanced 2016
Solution: