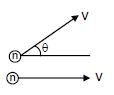
Q. In Maxwell's speed distribution curve, for N2 gas, the average of |relative velocity| between two molecules at 300 k will be :-
AIIMSAIIMS 2019
Solution:
$\left|V_{rel}\right|=\sqrt{V^{2}+V^{2}-2\left(V\right)\left(V\right)cos \theta}=2V\left|sin \frac{\theta }{2}\right|$
$\left\langle {V_{rel}} \right\rangle$=$\frac{\int^{^{^{\pi}}}_{_{_0}}2V1\left|sin \frac{\theta }{2}\right|d\theta}{\int^{^{^{\pi}}}_{_{_0}}d\theta}=\frac{4V}{\pi}$
$\left\langle V_{rel}\right\rangle$=$\frac{4}{\pi}V_{average}=\frac{4}{\pi}\sqrt{\frac{8RT}{\pi m_{0}}}=\frac{4}{\pi}\sqrt{\frac{8\times8.3\times300}{3.14\times28\times10^{-3}}}=606m/sec$