Q.
In homogeneous catalytic reactions, there are three alternative paths $A , B$ and $C$ (shown in the figure). Which one of the following indicates the relative case with which the reaction can take place?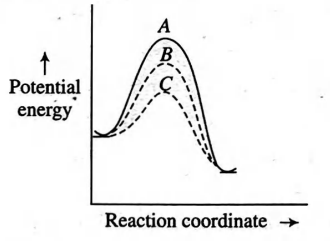
Surface Chemistry
Solution: