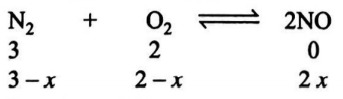Q. In a reaction vessel of $2\, L$ capacity $3$ mol of $N_{2}$ reacts with $2\, mol$ of $O _{2}$ to produce $1\, mol$ of $NO .$ What is the molar concentration of $N _{2}$ at equilibrium?
Equilibrium
Solution:
Solution: