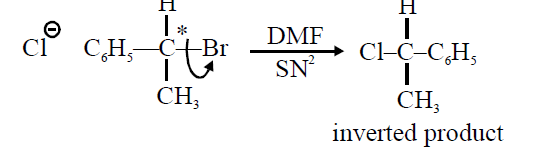
Q.
In a nucleophilic substitution reaction :
$R-Br + Cl^- \xrightarrow{DMF} R-Cl + Br^- ,$
which one of the following undergoes complete inversion of configuration ?
Solution:
In the compound of option B, bromine is attached to a primary carbon atom.
The substitution occurs via $SN _{2}$ mechanism which involves the inversion of configuration.
In the remaining compounds the bromine is attached to a secondary or tertiary carbon atom. In case of secondary carbon atom (options A and C), the
substitution occurs via a combination of $SN _{1}$ and $SN _{2}$ mechanisms. Hence, complete inversion of configuration is not possible.
In case of tertiary carbon atom, (option D) the substitution occurs via $SN _{1}$ mechanism which cannot result in complete inversion of configuration.