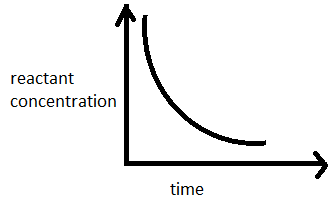
Q. In a first order reaction with time the concentration of the reactant decreases
BITSATBITSAT 2015
Solution:
For first order reaction $[ A ]=\left[ A _{0}\right] e ^{- kt }$
$\therefore $ The concentration of reactants will exponentially decrease with time.