Q.
In $1^{\text {st }}$ case, Carnot engine operates between temperatures $300\, K$ and $100 \,K$. In $2^{\text {nd }}$ case, as shown in the figure, a combination of two engines is used. The efficiency of this combination (in $2^{\text {nd }}$ case) will be :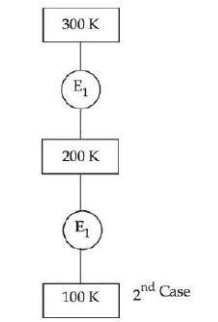
Solution: