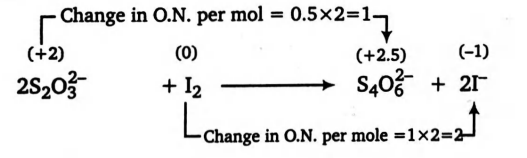Q.
If the molecular wt. of $Na_2S_2O_3$ and $I_2$ are $M_1$ and $M_2$ respectively, then what will be the equivalent wt. of $Na_2S_2O_3$ and $I_2$ in the following reaction?
$2 S _2 O _3^{2-}+ I _2 \longrightarrow S _4 O _6^{2-}+2 I ^{-}$
Solution: