Q.
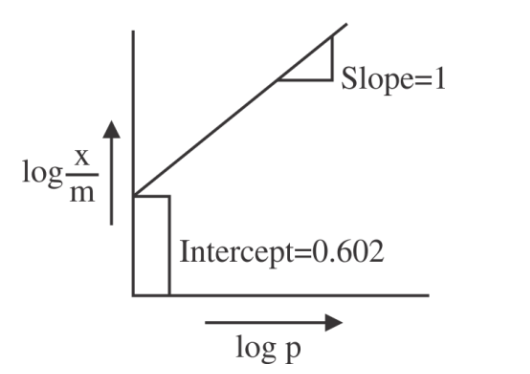
If the initial pressure of a gas is $0.03 \,atm$, the mass of the gas adsorbed per gram of the adsorbent is _____$\times 10^{-2} g$
Solution: