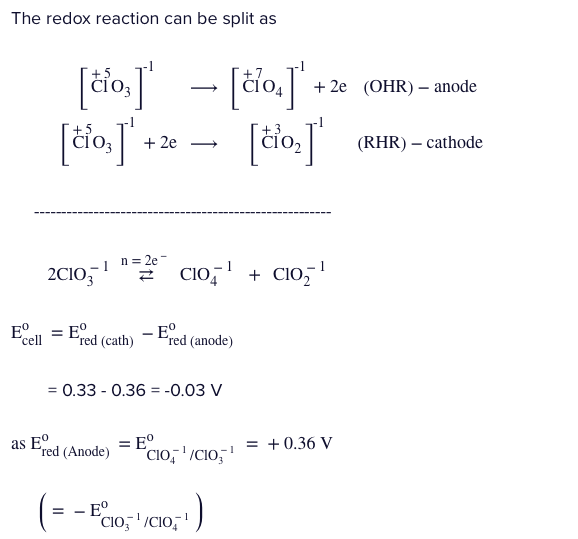
Q.
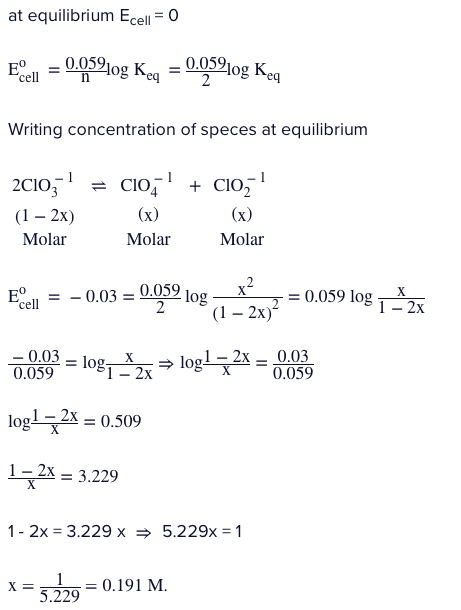
If $E_{ClO_{3}^{-} / ClO_{4}^{-}}^{0}=-0.36V\&E_{ClO_{3}^{-} / ClO_{2}^{-}}^{0}=0.33Vat300K.$ The equilibrium concentration of perchlorate ion $\left(ClO_{4}^{-}\right)$ which was initially $1.0M$ in $ClO_{3}^{-}$ when the reaction starts to attain the equilibrium,
$2ClO_{3}^{-}\rightleftharpoons ClO_{2}^{-}+ClO_{4}^{-}$
Given : $Antilog\left(\right.0.509\left.\right)=3.329$
NTA AbhyasNTA Abhyas 2022
Solution: