Q.
Ice crystallises in a hexagonal lattice. At low temperature at which the structure was determined, the lattice constants were $a=4.53\, \mathring{A}$ and $c=7.41\, \mathring{A}$. How many $H _{2} O$ molecules are contained in a unit cell? $\left(d_{\text {ice }}=0.92\, g\, /\, cm ^{3}\right) ?$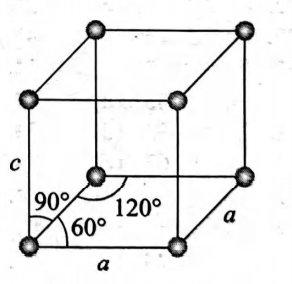
The Solid State
Solution: