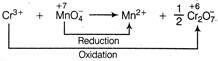
Q. How many mL of $ 0.125\text{ }M\text{ }C{{r}^{3+}} $ must be reacted with 12.0 mL of $ 0.200\text{ }M\text{ }MnO_{4}^{-} $ if the redox products are $ C{{r}_{2}}O_{7}^{2-} $ and $ M{{n}^{2+}} $ ?
ManipalManipal 2013
Solution:
Equivalents of $ C{{r}^{3+}}=3\times $ moles of $ C{{r}^{3+}} $ Equivalents of $ MnO_{4}^{-}=5\times $ moles of $ MnO_{4}^{-} $ Amount of $ C{{r}^{3+}}=0.125\times V\, $ milli mol $ =0.125\times V\times 3\, $ miliequiv. Amount of $ MnO_{4}^{-}=0.200\times 12.00\times 5 $ milliequiv $ \therefore $ $ 0.125\times V\times 3=0.200\times 12.00\times 5 $ $ V=32.0\,mL $