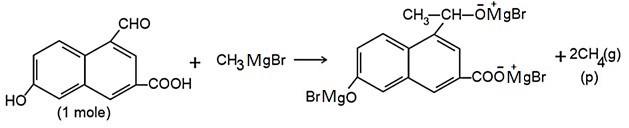
Q.

How many litres of gas $P$ is formed in above reaction at $NTP$ ?
(molar volume of gas at $NTP$ is $22.4 \, L$ )
NTA AbhyasNTA Abhyas 2022
Solution: