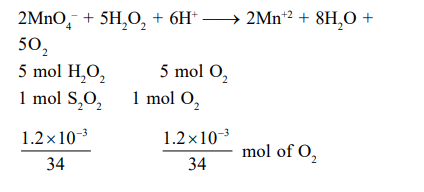Q. $H _{2} O _{2}$ is oxidised by $MnO _{4}{ }^{-}$in acidic medium to form $O _{2}$ and $Mn ^{2+}$. How many gram of $O _{2}$ are produced from $1.5 \times 10^{-3}$ moles of $MnO _{4}^-$ and $1.2$ milligram of $H _{2} O _{2}$ :-
Redox Reactions
Solution: