Q.
Given below are two statements :
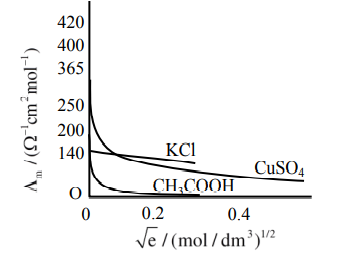
Statement I : The limiting molar conductivity of $KCl$ (strong electrolyte) is higher compared to that of $CH_3COOH$ (weak electrolyte).
Statement II : Molar conductivity decreases with
decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below :
Solution:
Ion
$H^+$
$K^+$
$Cl^-$
$CH_3COO^-$
$\Lambda^{\infty}_{m\,Scm^2/mole}$
349.8
73.5
76.3
40.9
So $ \Lambda_{ m CH _{3} COOH }^{\infty}=\Lambda_{ m \left( H ^{+}\right)}^{\infty}+\Lambda_{ m CH _{3} COO ^{-}}^{\infty}$
$=349.8+40.9 $
$=390.7\, Scm ^{2} / mole$
$\Lambda_{ m KCl }^{\infty}=\Lambda_{ m \left( K ^{+}\right)}^{\infty}+\Lambda_{ m \left( Cl ^{-}\right)}^{\infty}$
$=73.5+76.3$
$=149.3 \,S\,cm ^{2} / mole$
So statement-I is wrong or False.
As the concentration decreases, the dilution increases which increases the degree of dissociation, thus increasing the no. of ions, which increases the molar conductance.
So statement-II is false.
| Ion | $H^+$ | $K^+$ | $Cl^-$ | $CH_3COO^-$ |
| $\Lambda^{\infty}_{m\,Scm^2/mole}$ | 349.8 | 73.5 | 76.3 | 40.9 |