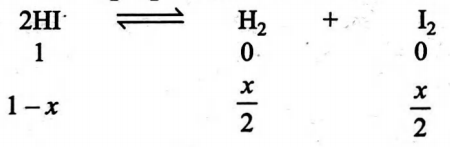Q.
For the reaction
$H _{2}+ I _{2} \rightleftharpoons 2 HI$
The value of equilibrium constant is $9.0 .$ The degree of dissociation of $HI$ will be
Equilibrium
Solution: